The most stable state for an atom is to have a complete outer electron shell like that of a noble gas. Shown below are the filled outer electron shells of four of the noble gases. The electron shell is indicated by the circle around the element name, and the electrons are represented by the black dots on the shell. In most cases a complete shell has eight electrons; however, for Helium the outer shell is complete with two electrons.

For some atoms, the simplest way to achieve a complete outer electron shell is to gain or lose one or two electrons. However the loss or gain of electrons has consequences on the charged state of the atom. An atom typically exists with no charge because its nucleus contains positively charged protons, surrounded by an equal number of negatively charged electrons. Protons and electrons each carry a single charge. When an electron is gained or removed, a single negative charge is added or subtracted from the atom; however, no corresponding positive charge is added or subtracted since no protons are transferred. Therefore a single negative charge is gained for each electron added and a single positive charge is gained for each electron that is lost. The resulting charged atoms are called ions. Ionic bonds are then created between ions of opposite charges that are attracted to one another through electrostatic interactions.
Elements belonging to the first two groups of the Periodic Table have only one or two electrons in their outermost electronic shell, and thus only need to lose one or two electrons to ‘expose’ a complete electronic shell of lower energy. On the other hand, elements belonging to groups 6 and 7 need to gain two or one electrons respectively to complete their outermost electron shell. Therefore it is not unusual to find elements from groups 6 and 7 with two or one negative charges, respectively, and elements from groups 2 and 1 with two or one positive charges, respectively.
The affinity for gaining electrons can be quantified using the term electronegativity. Electronegativity can be considered the electron-attracting power of an atom. The higher the electronegativity, the greater the desire to gain electrons. Based on this term, elements belonging to groups 6 and 7 have high electronegativities as they seek additional electrons to complete their electron shells, while elements belonging to groups 1 and 2 have low electronegativites. The difference in electronegativity between two atoms within an ionic bond is typically large.
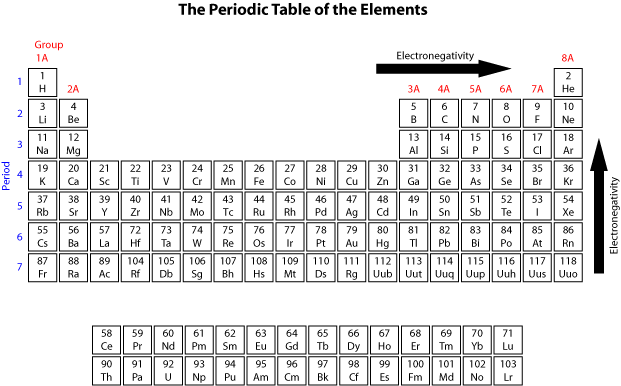
The strength of an ionic bond is dependent upon the environment of the ionic compound. In a vacuum, the strength of the bond is essentially immeasurable with an infinite strength. However let us consider two simpler examples – in air and in solution. In air, many ionic compounds are extremely stable and form elaborate crystalline lattices that maximize the number of ionic interactions between the individual ions. In aqueous (or water-based) solution, these crystals often disappear. Water is a polar molecule that has partially positively and partially negatively charged regions (as will be described more fully in the section on polar bonds). These partially charged regions have the ability to interact directly with individual ions. These interactions en masse can substitute for the interactions formed between ions. Thus, ionic bonds can be disrupted by water, leading to the separation of the ions.