Unlike ionic bonds, covalent bonds are often formed between atoms where one of the atoms cannot easily attain a noble gas electron shell configuration through the loss or gain of one or two electrons. In such cases, it is easier to ‘share’ valence electrons.
Electron ‘sharing’ occurs when the electrons in the outermost electron shell, or valence shell electrons, from one atom can be used to complete the outermost electron shell of another atom without being permanently transferred, as occurs in the formation of an ion. Electrons can only be so far from an atom before they are no longer associated with it; therefore, sharing electrons and forming covalent bonds restricts the maximum distance between two bonded atoms.
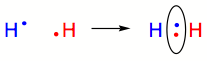
A complete outermost electron shell is the most stable state. Therefore atoms that bond covalently share their electrons to complete their valence shell. Let’s first use the example of Hydrogen (H). Hydrogen has one electron and requires one additional electron to have the same valence shell configuration as the noble gas Helium (He). The simplest way to achieve this is for two hydrogen atoms to share their single electron with one another. Shown below, each Hydrogen atom (H) and its single electron are depicted in either red or blue. When the two atoms share their single electrons (shown within the oval), the origins of the electrons are indistinguishable from one another. Therefore it appears that there are two electrons in the valence shells of both the red H and the blue H.

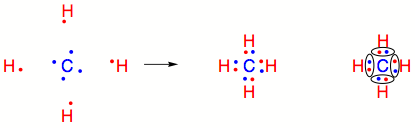
What about more complex examples? Carbon (C) has four valence electrons, and requires four additional electrons to have the same valence shell configuration as Neon (Ne). Forming a single covalent bond with a second carbon atom will not complete either atom’s valence shell. Therefore a Carbon atom can instead share each of its electrons with four separate Hydrogen atoms. This completes the valence shells for four Hydrogen atoms and one Carbon atom. In this example, one pair of electrons is shared between two atoms. The covalent bonds shown below are all called single covalent bonds.
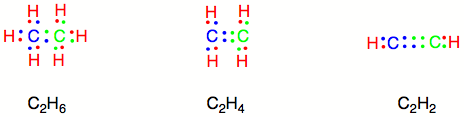
As one might surmise, there are a variety of ways of arranging electrons in covalent bonds. Different atoms connected by a covalent bond do not necessarily contribute equal numbers of electrons. Moreover an individual atom may contribute more than one electron to a covalent bond. Using Carbon and Hydrogen as examples again, consider the configurations of electron sharing required to create C2H6, C2H4, and C2H2.
The bonds formed by shared electrons are often simplified into lines between the bonded atoms. Each line represents one pair of electrons. Therefore the three compounds above can be redrawn as shown below.
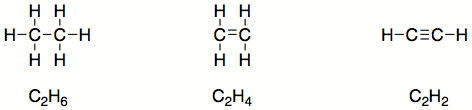
Using shared electrons to complete valence shells has some direct implications to the compounds. The valence shells will only be complete so long as the electrons are shared, which requires that the bonded atoms remain close to one another. Atoms within covalent bonds would lose their complete valence shells if they were separated. This is in contrast to ionic bonds; the valence shells of ions are not affected when ionic bonds are disrupted. Thus, covalent bonds are often very strong regardless of environment because the consequences to the valence shells would be the same in all situations.