1) Covalent bond between two atoms with unequal electronegativities results in unequal sharing of electrons. 2) Unequal electron sharing leads to partially positive and partially negative charges on opposite ends of the bond.
When the two atoms involved in a covalent bond both have equal affinity for electrons, the electrons in the bond are evenly shared between them. However if the two atoms have different affinities for electrons, or electronegativities, then the electrons are more likely to be found closer to the more electronegative atom. In this case, the more electronegative atom gains a partial negative charge, while the less electronegative atom becomes partially positive. These types of covalent bonds are called polar bonds because the electron distribution or charge is unevenly distributed or polarized. Partial charges are represented by lower case delta (+) δ+ or lower case delta (-) δ-.
![]()
A separation of partial charges within a polar covalent bond is referred to as a dipole. A dipole is represented by the symbol below, where the arrow points towards the partially negative atom, and the other end is at the partially positive atom.
![]()
An example of a polar covalent bond is that shown below of a double bond between carbon (C) and oxygen (O). Since oxygen is more electronegative than carbon, the electrons will spend more time around the oxygen atom giving it a partially negative charge while the carbon will become partially positive.
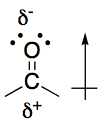
Some atoms retain lone (unbonded) pairs of electrons when they are part of a covalent complex. In the example shown above, the Oxygen atom has two lone pairs of electrons that are not bonded to other atoms. Each electron is denoted as a single black circle. These lone pairs are not shared between atoms and can also act as a partial negative charge.