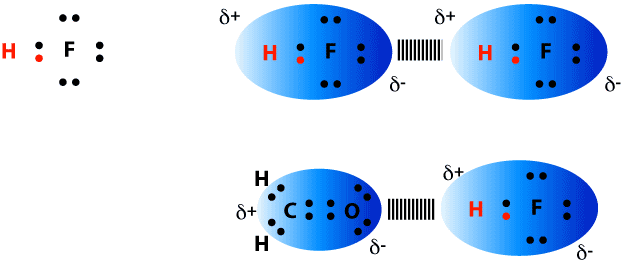
1) Bond formed between the positive end of a dipole generated between a Hydrogen bound to Cl, F, N, or O and the lone pair of electrons on a Cl, F, N, or O atom. 2) Extremely significant in biological molecules (protein folding, DNA structure).
Just as oppositely charged ions are attracted to one another and can form ionic bonds, the partial charges that exist at opposite ends of polar bonds can also interact with other partially charged molecules. Of particular importance is the dipole generated between a Hydrogen atom covalently bonded to any of the extremely electronegative atoms Fluorine (F), Oxygen (O), Nitrogen (N), or Chlorine (Cl). In these cases an extremely strong polar bond is generated where the hydrogen possesses a strong partial positive charge, and the F, O, N, or Cl possesses a strong partial negative charge. The partially charged hydrogen atom can then interact with another negatively charged atom. These bonds are called hydrogen bonds and are characterized by the interaction of a strongly partially positive Hydrogen to a lone pair of electrons on an extremely electronegative atom such as F, O, N, or Cl. In hydrogen bonds, the Hydrogen atom is the hydrogen bond donor, and the atom with the lone pair of electrons is the hydrogen bond acceptor.
Shown below are two different examples of hydrogen bonds (represented by the dashed line) formed with hydrofluoric acid (HF). In the first example, two HF molecules are shown aligning themselves to maximize the interactions of partially positive (Hydrogen) and partially negative (Fluorine) portions of the molecules. In the second example, the partially positive end of HF is shown hydrogen bonding to the partially negative portion of formaldehyde (H2CO).

Hydrogen bonds are similar to ionic bonds in that they are formed through the attraction of atoms possessing opposite polarities. However they are weaker in comparison because they are created through the interaction of partial charges instead of complete or full charges. Similar to ionic bonds, hydrogen bonds can be easily disrupted by polar solvents such as water for the same reasons as previously described.
If individual hydrogen bonds are so weak, then what is their significance? First, the cells of all living organisms are mainly composed of water. Because water forms hydrogen bonds with itself, other molecules that exist in the presence of water will either disrupt or interact with the hydrogen bonds formed between individual water molecules. Second, molecules essential for life such as proteins and nucleic acids possess a great capacity to form hydrogen bonds. These capabilities are essential for their function and structure. Third, while individual hydrogen bonds may not be considered strong, they are present in such huge abundance in the molecules of living organisms that their accumulated strength is tremendously significant.