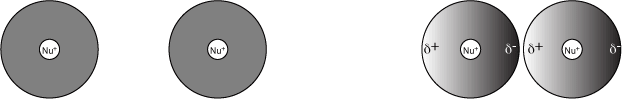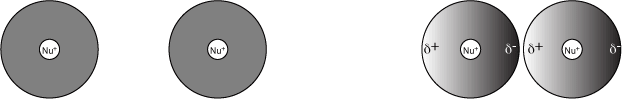
Previously dipoles were introduced in the context of polar or hydrogen bonds. In these cases the dipoles are permanently created through the extreme disparity in the electronegativity between the atoms in the bond. However dipoles can also be transiently induced.
If we consider the electrons around an atom in a molecule, the electrons are likely to be evenly distributed in space around it. However when placed in the presence of a charged molecule, the electrons surrounding that atom will be affected. If a negatively charged molecule is brought near, due to charge-charge repulsion the electrons will spend more time as far away from the charged molecule as possible. In contrast, if a positively charged molecule is brought near, the electrons will spend more time closer to the molecule. Both of these situations create a dipole within a single atom. However these dipoles are not permanent, since as soon as the charged molecule is moved away the electrons will distribute themselves evenly again.
Dipoles can also be induced when two non-polar molecules are brought close together. In this case however, instead of a strongly charged molecule pushing or pulling electrons away, the randomly arranged electrons will randomly shift themselves to maximize the potential positive-negative interactions that can be formed by these dipoles. In the left-hand figure below, the electrons are represented as a gray sphere of uniform intensity to indicate that the electrons are evenly distributed around the positively charged nucleus (Nu+). In the right-hand example, as the two atoms come closer together the electrons have become concentrated on the right side of each atom. Note that in the right-hand example, the electrons could have just as easily become concentrated on the left side of the atom; the side towards which they migrate is arbitrary. These types of bonds are also referred to as London disperson forces or van der Waals forces (interactions). Due to their transient nature, van der Waals interactions are considered the weakest forces. The strength of van der Waals forces correlates with the size of an atom; that is, a larger atom can form stronger Van der Waals interactions than a smaller atom.