DNA, or deoxyribonucleic acid, is the heritable material found in all cells. DNA provides the instructions to build, maintain, and regulate cells and organisms and is passed on when cells divide and when organisms reproduce. In this unit, the molecular structure of DNA and its packaging within cells will be examined. In 1953, using data obtained by Rosalind Franklin, James Watson and Francis Crick determined that DNA exists in a form known as the double helix. A helix is a winding structure like a corkscrew; DNA is known as a double helix because there are two intertwined strands within each molecule of DNA.
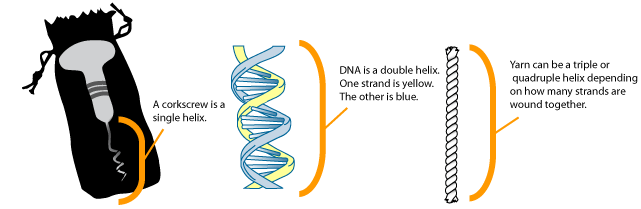
In the image above, a corkscrew is shown on the left, with the helical region labeled. The image in the center shows the structure of DNA. Note that there are two strands: one shown in blue, one in yellow. Other examples of a helix include yarn, a phone cord, or a spiral staircase.
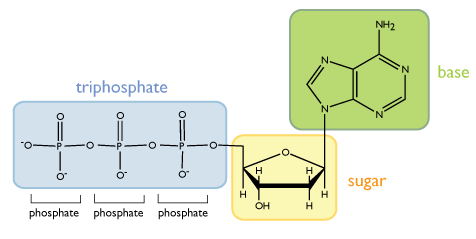
Each chain of the double helix is made up of repeating units called nucleotides. A single nucleotide is composed of three functional groups: a sugar, a triphosphate, and a nitrogenous (nitrogen-containing) base, as shown below. Note that in the figures drawn in this unit, each unlabeled vertex of a structure represents a carbon atom.

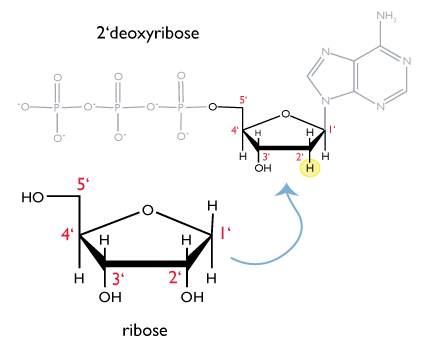
The sugar found in DNA is a variant of the five-carbon sugar called ribose. The structure of ribose is drawn below. Each carbon of ribose is numbered as shown. Because the -OH group on the 2’ carbon is missing in the form of ribose found in DNA, the sugar in DNA is called 2’-deoxyribose.
The second principle feature of a nucleotide is the triphosphate group attached to the 5’ carbon of the ribose group. In an aqueous environment, like inside the cell, the phosphate groups are negatively charged, as drawn in the figure above.
A free, unincorporated nucleotide usually exists in a triphosphate form; that is, it contains a chain of three phosphates. In DNA, however, it loses two of these phosphate groups, so that only one phosphate is incorporated into a strand of DNA. When nucleotides are incorporated into DNA, adjacent nucleotides are linked by a phosphodiester bond: a covalent bond is formed between the 5’ phosphate group of one nucleotide and the 3’-OH group of another (see below). In this manner, each strand of DNA has a “backbone” of phosphate-sugar-phosphate-sugar-phosphate. The backbone has a 5’ end (with a free phosphate) and a 3’ end (with a free OH group). In the structure below, each nucleotide is drawn in a different color, for clarity. 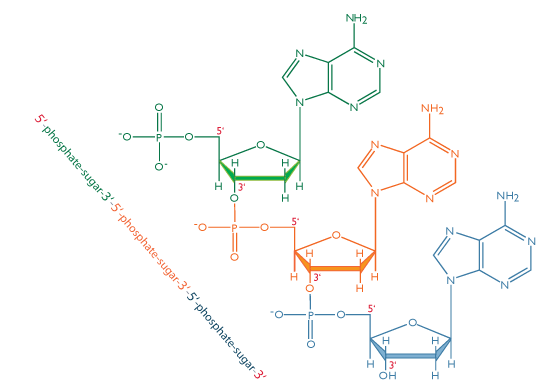
The third principle feature of a nucleotide is the base, which is attached to the 1’ carbon of the ribose. Although each nucleotide in DNA contains identical sugar and phosphate groups, there are four different bases and thus four different nucleotides that can be incorporated into DNA. The four bases are adenine, cytosine, guainne, and thymine, and their structures are shown below.
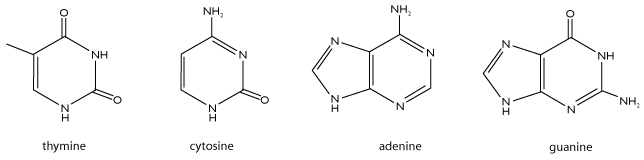
When these bases are incorporated into nucleotides, the nucleotides are called 2’deoxyadenosine triphosphate, 2’deoxycytidine triphosphate, 2’deoxyguanosine triphosphate, and 2’deoxythymidine triphosphate, respectively. We often shorten this notation to A, C, G, and T. Note that two pairs of bases have similar structures. A and G both have two carbon-nitrogen rings and are known as purines. In contrast, C and T have a single carbon-nitrogen ring and belong to a class of molecules called pyrimidines.
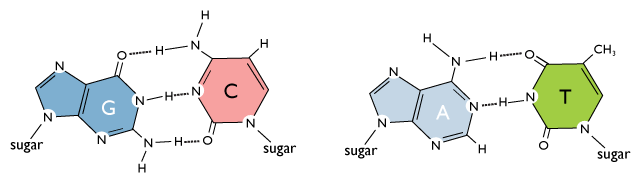
Hydrogen-bond interactions between the bases allow two strands of DNA to form the double helix. These interactions are specific: A base pairs with T, and C base pairs with G. This occurs via hydrogen bonds, which are shown with dotted lines in the figure above. If DNA were thought of as a spiral staircase, the base pairs would be the steps. The width of each “step” is approximately the same size, since a base pair always consists of one pyrimidine and one purine. The strands of DNA run anti-parallel, or in opposite directions: the 5’ end of one strand is paired with the 3’ end of the other. This is illustrated in the figure below.
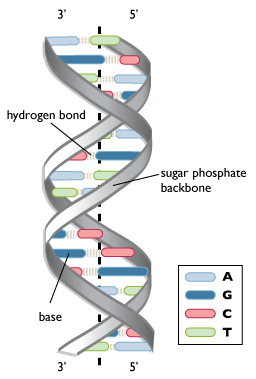
This structure places the non-polar bases of DNA in the center of the double-stranded molecule, surrounded by the charged phosphate groups. This has two functional consequences. First, remember that like charges repel each other. The double-helix structure, with negatively charged phosphates on the outside edges, allows the phosphates to be as far apart as possible. Second, the non-polar, uncharged bases are hidden in the center of the helix. The cellular environment is aqueous and therefore polar, so surrounding the non-polar bases with charged phosphates maximizes the solubility of DNA under physiological conditions. More information on polarity can be found in the tutorial on bonding.
Because of the specificity of hydrogen bonding, in the context of DNA A always pairs with T, and G with C. Therefore, if the sequence of one strand of DNA is known, the sequence of the other strand can be determined as well. In this way, if one strand of DNA is known to have the sequence 5’-ATGGCT-3’, the other strand must have the sequence 3’-TACCGA-5’. (Remember that the strands run antiparallel, so the 5’ end of one strand must be able to pair with the 3’ end of the other.) These strands are called complementary.